Minerals the Body Needs
Calcium, Iron, and Potassium are three of the most important minerals your body needs.
Free Download Below
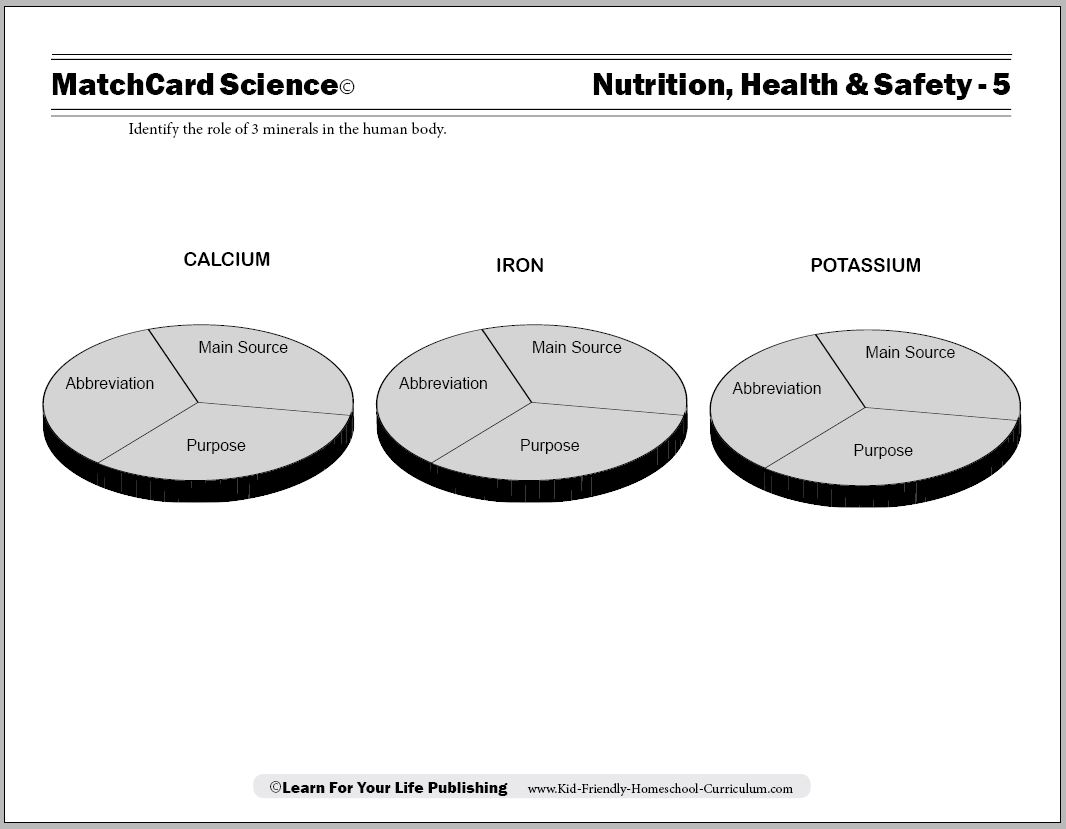

MatchCard Science Rocks and Minerals Worksheet
Objective: Identify the role of 3 minerals in the body.MatchCard: Download below.
MatchCard Information Pieces describing main function, source, and chemical abbreviation to calcium, iron, and potassium.
Hands on projects and other minerals also listed on this page.
Print the Nutritional Minearls MatchCard


Click image to go to download.
This is MatchCard #5 of the Nutrition, Health, and Safety Unit Study. Find more information on MatchCard Science below.
What Is a Mineral?
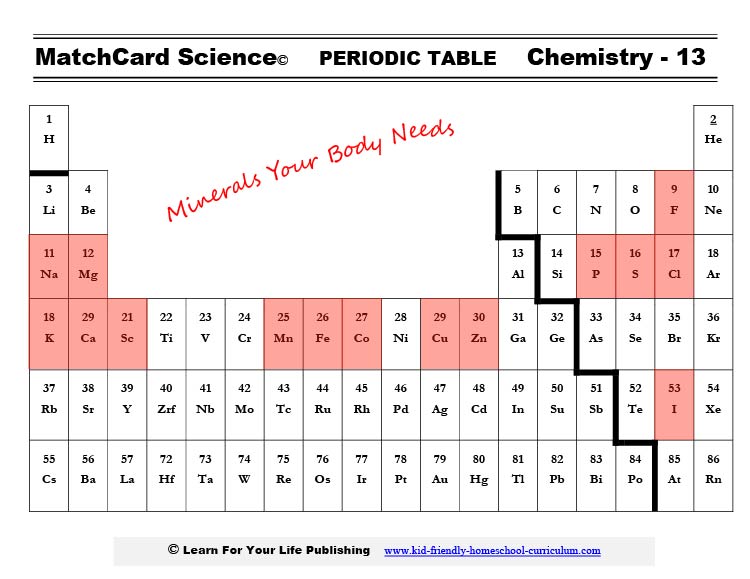
You may notice a + or ++ or even +++ symbol on some of these. That indicates that the atom lost some electrons and is now called an ion (an atom with a positive or negative charge.) If you haven’t studied it in chemistry, don’t worry about it now.
If you do remember studying this in chemistry, take note that these charged ions are extremely important to your body’s chemistry. Like no ions - no life.
To make things simple we are only going to discuss the most important function of three very important minerals. Like the vitamins, they have more than one function.
Also, they are needed in very small amounts. Vitamins and minerals are called micronutrients because only small amounts are needed. Check out the the comparison of grams vs milligrams.
Calcium
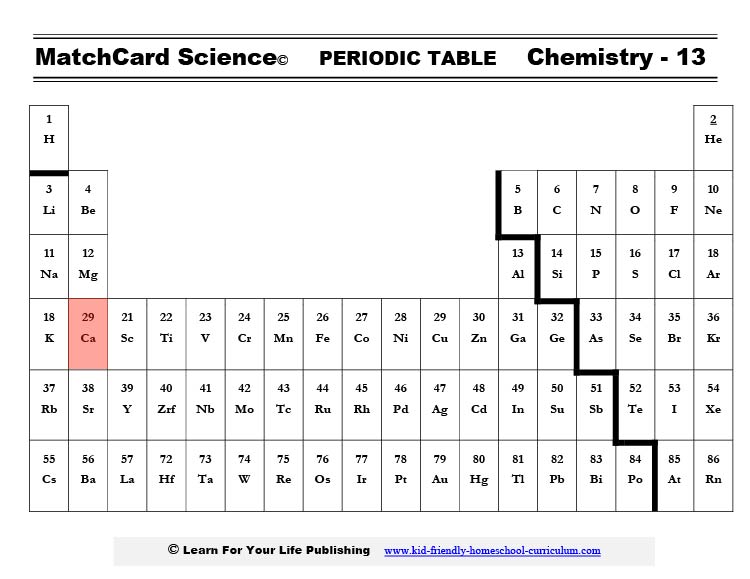
Facts About Calcium
If you find calcium on the periodic table, you will see that it is in the second column. It easily gives up two negative electrons giving it a positive charge. Hence the abbreviation Ca++.The major function of calcium is to build bones and teeth. Because it is an ion it also is involved in the contracting of all muscle cells (think heart - the most important muscle of all.) However, the bulk of the calcium in your body builds bones.
You might think bones are just a hunk of hard stuff, but actually they aren’t. If you look at bones under a microscope you will see holes somewhat swiss cheese. If a person has osteoporosis the holes become bigger and there is less bone. Broken bones result.
The biggest source of calcium in our diet is through dairy products.
Science Experiment With Calcium
Chalk It Up To Calcium
We are going to explore the chemistry of calcium a little more with this experiment. You will need three medium-sized kitchen glasses.- Fill each glass with vinegar (acetic acid.)
- Put a piece of chalk (calcium) in one.
- Put an uncooked egg still in its shell in another.
- Put a clean chicken bone in another.
- You will be able to watch the reaction of the chalk (calcium carbonate) with the vinegar (acetic acid) as it produces bubbles (carbon dioxide) in front of your eyes. The egg experiment may take several hours (the shell will dissolve) and the chicken bone takes several days (it will soften like rubber.)
- Meanwhile, you can reverse the experiment by putting baking soda back into the glass with the chalk.
Iron
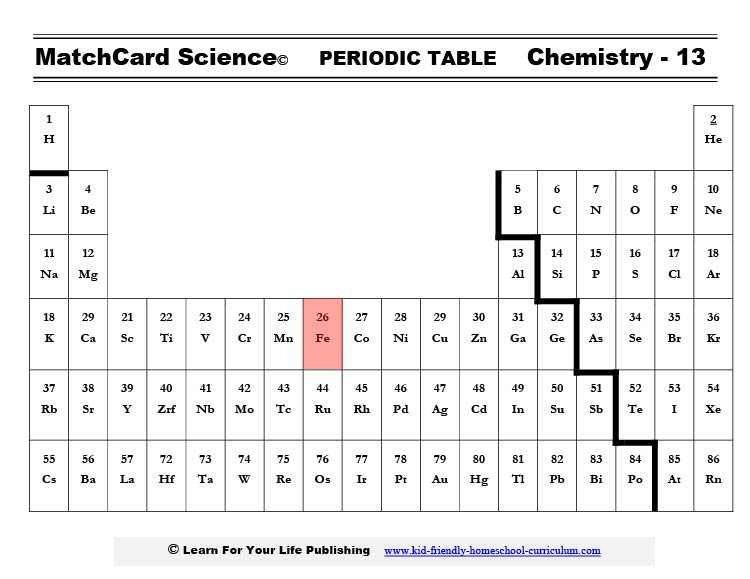
Let's Iron Out the Facts
Now if chalk makes our bones strong, you might think iron would really build tough tissues. It’s important, but it doesn’t do that.Iron carries oxygen in our blood. The red blood cells carry the oxygen we inhale through our lungs to all the individual cells of the body. No iron, no red blood cells, no life. That’s why losing all your blood is a really bad thing.
The chemical symbol for iron is Fe which is short for ferrous. This isn’t like the ferris wheel at the fair, but the French word for iron. I know, it’s confusing but maybe the ferris wheel is made out of iron and can be easier to remember.
What’s your best source of iron? Meat. Liver is particularly high. If you are a vegetarian plan to eat lots of spinach. Also, many cereals are iron fortified and you will do a project on that shortly.
Science Experiment: Where Does the Red Come From
When iron mixes with oxygen it turns red. That is why pictures of the body show the veins with blue, unoxygenated blood and the arteries with red, oxygenated blood.Remember iron + oxygen = red.
Do you remember seeing that any place besides anatomy books? Yep, rust. When iron is exposed to oxygen over long periods of time it rusts. Rust is red.You can do this experiment to show the same thing. We will do it in water which speeds up the process.
Add iron nails and fine steel wool to a glass. Cover it with water. Watch it for several days.
You can add other metal objects and see which contain iron.
Science Experiment: Iron in My Cereal
Many breakfast cereals are fortified with iron. This experiment will help you determine which of your favorite cereals contain the most iron. You will need three bar magnets to carry out this project.- Pour one cup of three different breakfast cereals in three different bowls.
- Add just enough water to cover the cereal.
- Let it set several hours.
- Stir to make it mushy. (Yum!)
- Hold a magnet with tweezers. You can use the tip of your fingers to hold the magnet but it is not as accurate.
- Put a bar magnet in each bowl and stir for 5 minutes (time it so it is the same.)
- Pieces of iron will be on the magnet. You may wish to use a magnifying glass to look at the amount.
- Repeat with other two cereals.
- If you have an accurate scientific scale, you can weigh the magnets before and after stirring the cereal to determine the exact amount of iron. Otherwise compare the amounts of iron visually.
Potassium
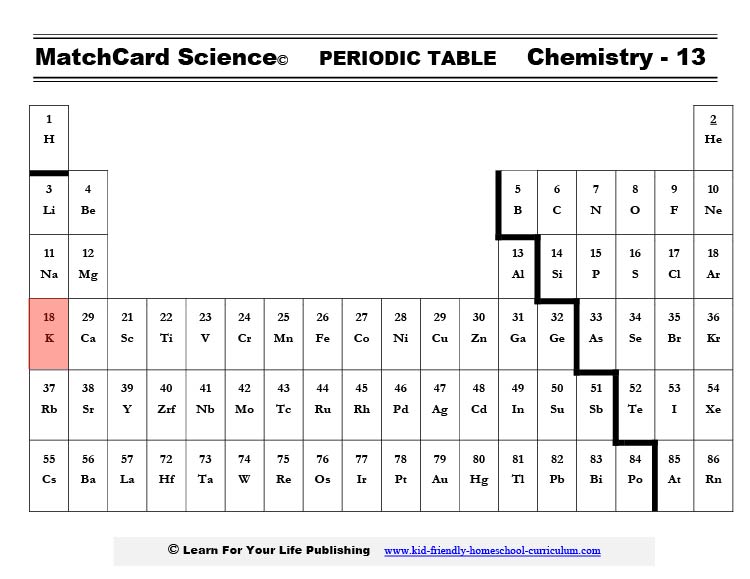
Potassium Facts: It's A Salty Subject
Potassium is an electrolyte. That is a salt that helps to regulate the amount of fluid in the blood stream and in every cell of the body.Another important salt is sodium. (Sodium chloride is table salt.) We don’t actually think of sodium as a nutrient because most of us have TOO MUCH sodium.
Potassium is another electrolyte/salt that is also used in every single cell of the body. When sodium leaves a cell potassium goes into it and vice versa. The two of them work together to keep the amount of fluid and electrolytes in perfect balance.
Potassium is abundant on the earth. About 2% of the earth’s crust is made of potassium. So we get it readily from fruits and vegetables.
When people sweat or vomit they lose potassium. Not a very pleasant subject, but at times like that electrolyte replacement is important.
The symbol for potassium is K. That’s because the word for potassium was kalium in Latin. If you check out the placement of potassium on a periodic table you will see it is on the first row so it easily gives up one electron. That gives it one additional proton charge and makes the ion K+.
Potassium Electrolyte Drink

What you may not know is that it also has a lot of sugar, calories, and preservatives in it. Yeah electrolytes: boo artificial sweeteners.
You might want to make this electrolyte drink for the next time you are sweating in the garden (you do help in the garden, don’t you?)
- 3 cups of coconut water
- 3 cups of orange juice (or other citrus juice)
- 1/4 tsp salt substitute (potassium iodide)
Potassium Demonstration: The Electro Whoa!
Did you notice the prefix electro in electrolyte?It conducts electricity. Most experiments with potassium are not very safe. In fact, they are electrofying. I mean, they make fireworks out of potassium.
You can check the internet for some cool potassium experiments that I don’t recommend you do in your living room. Or with your baby sister watching you.
L Here’s one you and your mom can do out on the front yard. Adult supervision required anytime you are using fire.
- Sprinkle some potassium iodide (that salt substitute) in a pie pan.
- Pour a small amount of Heet antifreeze into the pan.
- Light with a long lighter. (It will have a large flame.)
All the Minerals Your Body Needs

- Chloride
- Cobalt
- Copper
- Fluoride
- Iodine
- Iron
- Magnesium
- Manganese
- Phosphorus
- Potassium
- Selenium
- Sodium
- Sulfur
- Zinc
MatchCard Science
How To Use MatchCards
Download the FREE MatchCard Science Instructor's Guide and see how MatchCards can make building their science knowledge base fun.
Nutrition, Health, and Safety Unit Study

See more about the Nutrition, Health, and Safety Unit Study.
12 Science Unit Studies

Chemistry is only one of twelve complete unit studies for kids in 3rd to 8th grade.
Comprehensive objectives, hands-on projects, suggested science fair experiments, and the fun game-like MatchCards keep them interested in learning science. See all twelve MatchCard Science Unit Studies.
About Our Site
Hands-On Learning